When you start using a new battery, the aging process begins immediately. A battery consists of usable energy, a rechargeable part, and a part that permanently degrades—known as ‘rock content’. This unusable part increases as the battery ages, reducing capacity. It’s a natural process that affects every type of battery.
In practice, this means most batteries do not retain their full capacity throughout their lifespan. This directly impacts the performance of the device they power. Manufacturers often design their devices based on a battery performing at 100%, but in reality, this capacity quickly drops below optimal levels. Replacement is recommended once capacity drops to 80%, a point that arrives sooner than many expect.
High capacity in a battery is only valuable if it can efficiently utilize this energy. This brings us to the concept of internal resistance. Low internal resistance allows the battery to effectively deliver its stored energy. It’s like a water tap: the less resistance, the better the flow. High internal resistance can cause a device to shut down prematurely because the battery cannot deliver energy quickly enough.
Interestingly, despite their aging, lead-acid batteries maintain low internal resistance and perform well in delivering short, powerful bursts of power. Alkaline and zinc-carbon batteries, however, have higher internal resistance, making them less suitable for high-energy devices.
Self-discharge is a phenomenon that affects every battery and varies greatly between chemistries. For example, lithium and alkaline batteries are better at retaining their charge compared to nickel-based batteries. The latter can experience significantly more self-discharge, necessitating frequent recharging. Self-discharge increases with the age of the battery, the number of usage cycles, and ambient temperature.
In most portable devices, the battery is switched off before it is completely empty to prevent damage and maintain a reserve for self-discharge. For instance, mobile phones and laptops usually shut down at a voltage of 3V per cell. In hybrid cars, the full capacity of the battery is never used; they operate within a specific state of charge to maximize lifespan.
The life of a battery is affected by a complex mix of factors, including ageing, internal resistance, self-discharge and the management of discharge depth. While we cannot completely stop these processes, we can implement strategies to extend the life of our batteries, such as regular maintenance and avoiding extreme temperatures. By better understanding how batteries work, we can optimise our reliance on them and maximise their lifespan.
Elfa is more than a supplier of batteries and accumulators. We are a partner that thinks with you and helps you find the best energy solution for your business. Whether you need lithium-ion batteries for your electric vehicles, emergency power supplies, or smart devices, or other types of batteries for specific applications, we have it all. We are here to support you with our knowledge, experience, and service. Contact us today and let us know what we can do for your business!
In the fast-changing world of technology, home batteries have become a hot topic of discussion; this is especially true for homeowners striving for a sustainable and energy-independent household. The home battery allows excess energy from solar panels to be stored; it can then be used at times when the sun is not shining. This gives households more control over their energy consumption and at the same time allows them to reduce their carbon footprint.
While the technology seems like a promising step towards a greener future, it is essential to take (fire) safety around home batteries seriously. In this blog, we discuss not only the benefits of home batteries , but also how to integrate them safely and efficiently into your household.
Home energy storage systems are an integral part of the shift to renewable energy sources. Essentially, the home battery stores excess energy produced by solar panels during the day.
This stored energy comes in handy when the sun goes down; it removes households’ dependence on the traditional electricity grid. Besides energy independence, this also means savings on energy bills and reduced environmental impact.
While home energy storage systems are a promising technology, it is important to understand that they are not without risk. These batteries are often made of lithium-ion technology; this technology is known for its efficiency in energy storage, but also for its possible ‘dangerous’ properties.
Overheating, short circuits, manufacturing defects or even mechanical damage can lead to a ‘thermal runaway’. This process occurs when the temperature inside the battery cell has exceeded a certain point; that is, the heat generated exceeds the heat dissipated
In practice, this means nothing more than that the battery may catch fire. In a domestic environment, such a fire can spread rapidly, highlighting the need for precautions.
To minimise the earlier-mentioned fire risk, several steps can be taken to significantly reduce the risk of fire in home batteries:
Always have home batteries installed by a qualified installer. These professionals are aware of all applicable standards and regulations. They will also ensure that the installation is safe and efficient.
Place the energy storage system in a well-ventilated, dry and cool place, preferably outside the house. Avoid installation near combustible materials to further reduce the risk.
Consider using fire-resistant materials or a fire separation system around the home battery. This can prevent any fire from spreading quickly; this can save you valuable time in getting the situation under control.
Make sure you have a functioning cooling system to prevent overheating. Maintaining a safe temperature range is vital to minimise the risk of fire. Most home batteries and inverters use natural cooling. However, if the temperature becomes too high, the Battery Management System (BMS) will reduce the power output. In the residential market, a cooling system will rarely be present. One important way to prevent heat generation is to design the system so that it never runs at full capacity, but perhaps only at 50%. This not only benefits the lifespan, but also significantly improves safety.
Regular maintenance and inspection of the energy storage system are crucial. Any sign of wear or damage should be addressed immediately to avoid potential problems. The downside, however, is that these systems are often maintenance-free. This means you are completely dependent on the Battery Management System (BMS), especially in the case of lithium batteries. This makes it virtually impossible to perform maintenance on a residential ESS system.
Implementing a home battery in your household offers many benefits, ranging from energy independence to lower energy costs. However, it is vital to integrate this technology in a safe and responsible manner.
With expert installation, careful site selection, proper temperature control, regular maintenance and fire safety awareness, you can enjoy all the benefits of a home energy storage system with peace of mind. This way, you not only contribute to a greener future, but also take into account a safe living environment for you and your fellow residents . Safety is paramount in the energy revolution of the 21st century; it is up to all of us to make this revolution not only sustainable, but also safe for everyone.
Would you like more information on the safety of energy storage systems, or help choosing the right storage system for your application? Our experts are ready and happy to help. Fill in your details in the contact form below and we will contact you soon.
The concept behind electric cars that act as home batteries is derived from the idea on bidirectional charging; this is also known as Vehicle-to-Home technology (V2H) or Vehicle-to-Grid technology (V2G).
This concept means that electric cars are not only able to receive electricity from the general power grid to charge, but they also have the ability to deliver electricity back to that same grid or even to your own home, at times when required.
Thanks to bidirectional charging, electric vehicles are transformed into versatile energy storage systems. Such systems can both absorb and release energy, depending on the situation and the owner’s needs.
Electric cars become not only an efficient means of transport, but also an important part of the larger power grid and home energy infrastructure. This opens the door to innovative ways of managing energy and enables more effective use of renewable energy sources.
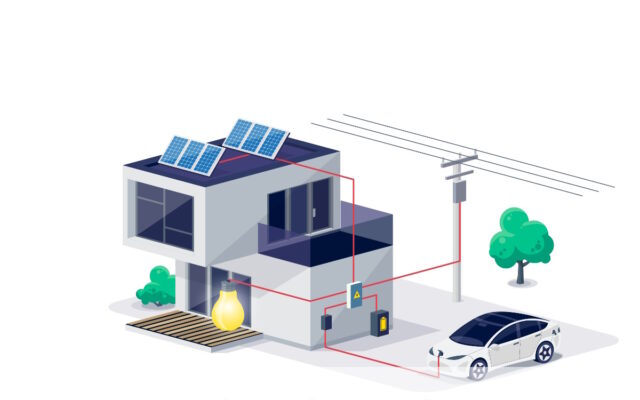
Suppose you are considering using an electric car as a home battery, then it is good to know what this can bring. What are the biggest benefits of storing power in your electric car? We explain three benefits in relation to bi-directional charging.
One of the most obvious advantages of this concept is the ability to use excess energy stored in your electric car as emergency power during any power outage. The car can act as a reliable source of backup power for essential appliances in these situations.
Electric cars can store energy during off-peak hours, when electricity costs are low, and use that energy during peak hours, when rates are higher. This can result in significant savings on your energy bills.
Using electric cars as home batteries can reduce the load on the electricity grid, especially during peak hours. This can help the power grid operate more efficiently and reduce the demand for fossil fuels.
The disadvantages of using electric cars as home batteries are less well known; to date, not much research has been done on this concept. We highlight two disadvantages related to bidirectional charging.
Implementing bidirectional charging requires advanced technology and special equipment like a bidirectional charging system; this can increase installation costs. At the moment (September, 2023), not all electric cars are V2X (vehicle to everything, and therefore also V2G and V2H) capable. But this is likely to change soon.
The effect of bidirectional charging on the battery is not yet entirely clear. On the one hand, the battery seems to ‘wear out’ faster as a result of extra charging cycles. On the other hand, frequent use may actually contribute to longer battery life. The effects of this are being studied by several countries.
Using your electric car as a home battery requires some planning to ensure the battery is full in the evening. If you want to do this with self-generated solar energy, you need to be home during the day to charge the battery. You may have to top up the battery in the morning if you use it as a home battery, and this may incur costs such as EUR 0.60 per kWh when using a fast charger.
The future of electric cars as home batteries seems promising, however, some challenges still need to be overcome. With further technological developments and the growing acceptance of bidirectional charging, electric cars could play an even bigger role in the transition to renewable energy sources in the future.
The idea that your car is not just a means of transport, but also a home energy storage system, opens the door for a more decentralised and resilient energy system. Besides the financial benefits for individual users, it can also contribute to a greener and more sustainable future for all of us.
Conclusion: The concept of an electric car as a home battery is interesting and promises to contribute to a more sustainable future. Although this technology is still developing, it is certainly worth following its progress within the world of renewable energy. At present, however, it still poses a reasonable challenge because of the required planning if you wish to charge with solar power at home, possible additional costs and the accelerated depreciation of the battery. It is a promising idea that may become more feasible in the future as technology improves and infrastructure develops.
Want to know more about what Elfa can do for your business in terms of battery and lighting solutions? Our experts are ready to answer all your questions. Fill in the form below and we will get in touch with you as soon as possible.
Have you ever wondered what a saltwater battery exactly is? It is an alternative form of energy storage also known as a flow battery or a redox flow battery. The saltwater battery utilizes electrolyte fluids based on salt solutions.
The saltwater battery offers numerous advantages: scalability, long-duration energy discharge, and the ability to independently size power and capacity. The operation of the saltwater battery is achieved through redox reactions between electrolyte solutions with different salt concentrations, which are separated by a membrane.
These types of batteries can play a significant role in supporting sustainable and reliable energy supply systems.

Sodium-ion saltwater battery 24V 112Ah
The FZSONICK sodium-nickel battery made its debut in 1999 as an alternative technology with significant potential in the field of energy storage. Unlike regular saltwater batteries, the FZSONICK battery utilizes sodium and nickel chloride as electrochemical materials.
This type of FZSONICK battery is also known as a Na-NiCl2 battery or a Zebra battery. Thanks to its unique composition and properties, this Swiss-made battery opens new perspectives in the world of energy storage.
The FZSONICK sodium-nickel battery utilizes an electrochemical reaction process inside the battery cell. During the charging of the battery, electric energy flows through the cell, causing sodium ions to move from the sodium anode to the nickel chloride cathode. When discharging the battery, the sodium ions are returned to the sodium anode, releasing electrical energy.
The FZSONICK sodium-nickel battery offers several advantages over the saltwater battery. Some of these advantages include:
High Energy Density
Compared to saltwater batteries, sodium-nickel batteries generally have a higher energy density. This means they can store more energy in a smaller form factor, making them beneficial in situations with limited space.
Fast Charging Times
FZSONICK batteries can be rapidly charged, which is useful in scenarios where quick energy uptake is required.
Long Lifespan
FZSONICK batteries typically have a longer lifespan. Additionally, they can withstand a large number of charge-discharge cycles, making them a durable and cost-effective solution compared to saltwater batteries.
Temperature Resistance
The FZSONICK batteries perform well at high temperatures, making them suitable for applications where heat may be a factor.
Safety
Similar to saltwater batteries, FZSONICK systems are extremely safe to use. The product is entirely free from risks of fire, explosion, and the release of harmful gases. This makes the FZSONICK sodium-nickel battery an ideal choice for businesses that prioritize the highest safety and reliability standards.
While saltwater batteries have excellent properties for large-scale energy storage, it is essential to stay updated on alternative technologies. If you wish to receive more information about the FZSONICK alternative to the saltwater battery, our experts are ready to assist you!
Please, fill in the contact form below, we will get in touch with you promptly.
Lead-acid batteries are widely used because of their reliability, simple charging procedures, low self-discharge, and relatively low costs. They are ideal for applications where a large amount of power is needed for a short time, such as when starting combustion engines.

Lead-acid battery from FIAMM
On the other hand, lithium-iron-phosphate (LiFePO4) technology has several advantages over lead acid, including higher energy density, longer lifespan, and lower weight. In addition, they are better resistant to damage from deep discharge. Advantages of LiFePO4 batteries:
So the choice between these two types of batteries depends on the specific needs of the application, including factors such as cost, weight, lifespan, charging time, and safety.

LiFePO4 accu PBQ
Are you looking for a suitable battery for an industrial application, but don’t know whether a lead-acid or lithium-iron-phosphate battery is more suitable for you? Please contact us by filling out the form. We would be happy to advise you!
The first factor influencing battery life is the type of battery chosen, i.e. the electrochemistry. For example, lithium batteries last about seven times longer than alkaline, depending on which type of lithium and the brand of battery. More information on different types of primary batteries can be found here.
The ambient temperature in which the battery is stored and used has a great influence on the battery life. For example, primary batteries are generally best kept cool and dry. Furthermore, primary batteries have an ideal operating temperature at which they can provide the most energy. For alkaline batteries l gt is around 20 °C. When the ambient temperature is significantly higher or lower the performance of the battery will be less. Lithium batteries, on the other hand, can better withstand different temperatures.
Alkaline batteries are ideal when the power used is usually low, such as devices that do not use much power during operation or are used periodically, such as remote controls or radios. Lithium batteries are generally better at handling peak current and can have a higher energy density. Therefore, these batteries are widely used among others in medical devices, IoT applications and smart meters.
Want more information about primary battery life, or help choosing the right battery for your application? Our experts are ready and eager to help you. Please fill in your details in the contact form below and we will get back to you quickly.
The disadvantage of this type of energy is that it is only available when the sun is shining and the wind is blowing. So that requires a different approach. The world will start using “smart devices”; that are devices that turn on when a lot of power is available. In a similar way we will be charging the electric car and the industry will also have to deal with power. The chemical factory will soon be running at peak production on a windy day.
But in addition, it will continue to be necessary to store electricity. Eneco recently built the largest battery in Europe in northern Germany. The thing is seventy meters long and reportedly cost 30 million euros. The wind energy that can be stored in it is just enough to provide electricity to 5,300 households for one 24-hour period. This mainly proved that this solution is too expensive and extensive for the power supply.
However, Elfa expects that large batteries will soon be a part of the electricity grid. After all, these mega batteries are useful for keeping the electricity grid in balance. The frequency of the power grid must remain constantly exact at fifty hertz. Nowadays, gas power plants can still be shut down when the wind is strong, or fired up on cloudy days. But in the near future, those power plants will not be there. Batteries can then provide a buffer that provides stability. Also the batteries from cars for that matter. Soon we will have millions of electric cars in the Netherlands. These cars are stationary more than 90% of the time. At peak times, owners can choose to, power from the car battery back into the grid.
If we want to store wind – and solar power for a long time, converting it to hydrogen seems to be the best option now. The green power splits water into oxygen and hydrogen through a process of electrolysis. Some energy is lost in the process, but the obvious advantage is that hydrogen gas can then be stored in tanks indefinitely. When burned, the energy is released again, but without CO2. Natural gas combustion does. The rest product is pure water.
Plans are currently being developed for an energy island in the North Sea with a hydrogen factory that will convert power from offshore wind farms into the clean gas. Factories can use hydrogen as an energy source or feedstock. And through the existing natural gas grid, it can even be brought to our homes. Cars can drive on it. And those hydrogen cars can act as power factories that supply electricity back into the grid at peak times. We believe in it and in the meantime we still see numerous applications for the battery.
Function of the batteries
All electrical power on the ISS is generated through the station’s solar panels, which convert sunlight into electrical energy. However, during times when the ISS goes through “orbital night,” the solar panels can no longer produce energy. As such, it is necessary for the ISS to store energy in batteries, which it can then use to power its systems during periods of darkness. Every 1.5h the ISS makes an orbit around the earth, 45 minutes of which is in sunlight. During this period, the batteries are charged by the solar panels and the batteries are discharged while feeding the station’s loads during the 45-minute period of darkness per lane.
The nickel-hydrogen (Ni-H2) batteries
The ISS has eight separate power channels in total, with each channel having three batteries – although one battery is considered a “series” of two separate battery units connected together, which actually amounts to six batteries per channel, and thus 48 batteries on ISS in total. Each of the old batteries is of the nickel-hydrogen (Ni-H2) type, which have generally always been used in space applications because of their long life, as they can withstand a large number of discharge cycles without major deterioration. In addition, Ni-H2 batteries are not susceptible to overcharging and countercurrent, giving them good safety properties.
However, one disadvantage of Ni-H2 batteries is that they are prone to “battery memory,” where the battery can lose some of its capacity if it is not fully charged and discharged during each cycle. For this reason, regular “battery conditioning” is performed on the ISS to prevent battery memory. Each of the station’s Ni-H2 batteries consists 38 individual cells (76 cells per string of two batteries), with each cell consisting of a pressure vessel containing gaseous hydrogen stored to 1,200 psi, which is generated during the charging process itself. The oldest batteries at the station are now about 10 years old and are reaching the end of their design life.
Lithium-ion (Li-ion) batteries
This means that replacement batteries are needed to maintain the ISS until its current planned retirement date of 2024. However, Ni-H2 batteries are now considered old technology, as most of the station’s systems were designed in the late 1980s and early 1990s. The ISS program has therefore decided to modernize the station’s batteries during the replacement process by switching to modern lithium-ion (Li-ion) batteries. These battery types operate through lithium ions that move between electrodes during the charging process, rather than pressurized hydrogen gas as used in Ni-H2 batteries.
As a result, Li-ion batteries are much lighter and smaller than Ni-H2 batteries because they do not require pressure vessel containers to store hydrogen gas, which means that Li-ion batteries have a very high energy density compared to Ni-H2 batteries. This has many advantages for the ISS program, because it means that only a single Li-ion battery can replace the function of two of the previous Ni-H2 batteries. This means that only half the number of Li-ion batteries (24) are needed to replace all of the station’s Ni-H2 batteries (48), which also halves the number of launches required. Li-ion batteries are also not sensitive to battery memory, so there is no need to condition the battery. However, Li-ion batteries have some drawbacks, namely the fact that they are much more sensitive to overcharging, which must be prevented through battery management and protection systems. In addition, Li-ion batteries typically have a shorter life span than Ni-H2 batteries because they cannot endure as many charge/discharge cycles before experiencing noticeable degradation. However, the ISS Li-ion batteries are designed for 60,000 cycles and a lifetime of ten years. In addition, they will include cell balancing and adjustable charge voltage technology to maximize their lifetime.
Li-ion batteries have experienced notable problems in the past, in the form of overheating and “thermal runaway.” The Li-ion batteries that will be used on the ISS, while manufactured by the same company (GS Yuasa), were designed with lessons learned from the problems, and have passed space certification tests. In particular, ISS Li-ion batteries include two schemes against thermal runaway, voltage and temperature monitoring of individual cells, circuit protection and fault isolation of individual cells, and thermal heat barriers between cell packs.
In terms of construction, each ISS Li-ion battery contains 30 individual cells, packed in a box that retains the same dimensions and mounting interfaces as previous Ni-H2 batteries, but with a significantly reduced weight (430 pounds instead of 740 pounds). A single Li-ion battery replaces the functions of two Ni-H2 batteries, but since two Ni-H2 batteries are connected in a “string” and are considered one battery, this means that adapter plates are also needed. This is to connect the single Li-ion battery to the existing connections for the unnecessary second battery in each string, thus completing the circuit.